Key gene blocking ‘spillover’ of avian flu to humans discovered
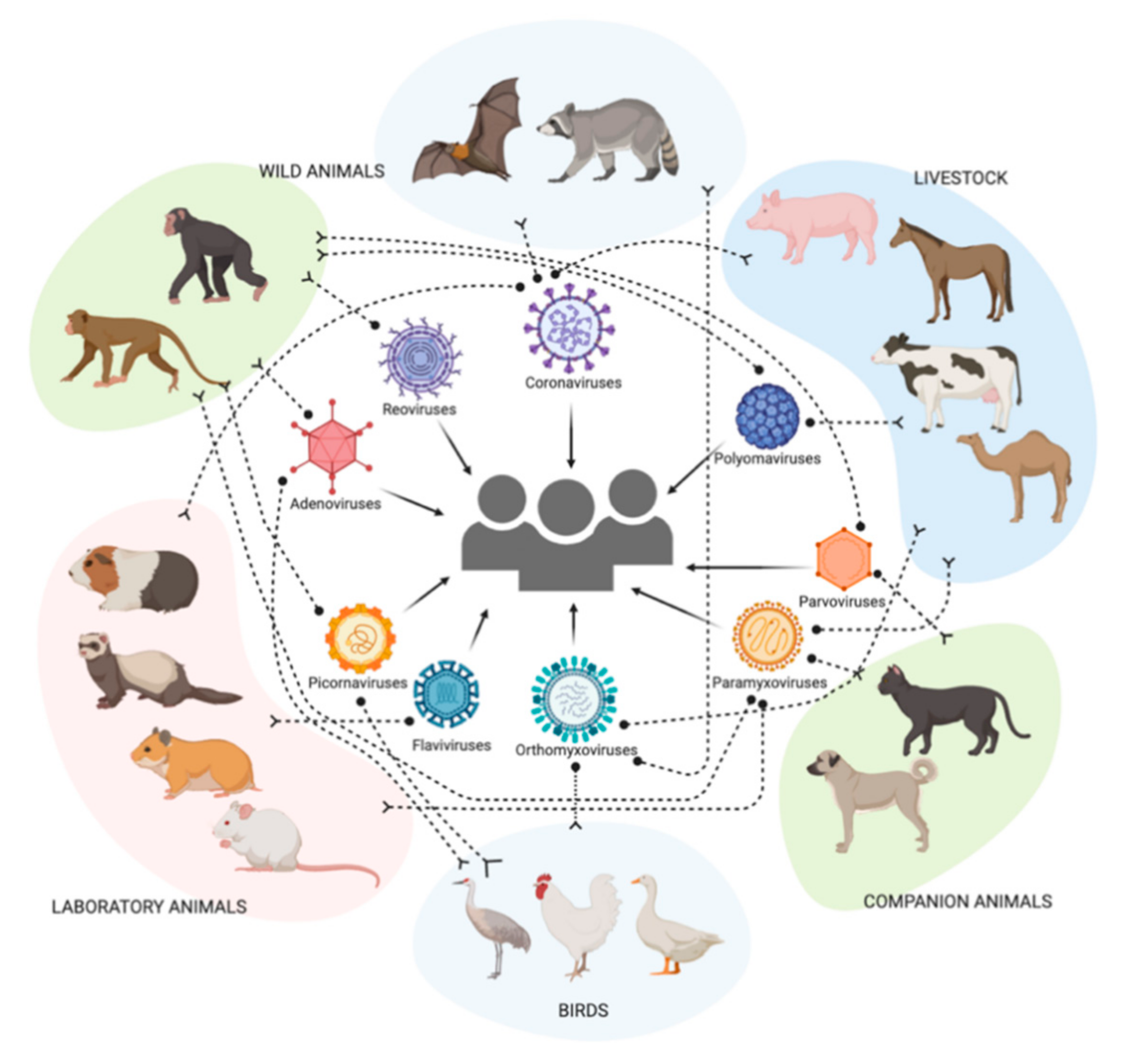
Scientists found that avian flu strains resistant to BTN3A3 increased around the time of spill over events in humans
Understanding the genetic make-up of currently circulating avian flu strains may offer one of the best lines of defence against widespread human transmission. This is according to new research which has found a key human gene responsible for blocking most avian flu viruses from spilling over into people.
According to a press statement by Glasgow University, the latest international study into the pandemic potential of avian flu, which is led by scientists at the MRC-University of Glasgow Centre for Virus Research (CVR) and published in Nature, identified the human gene BTN3A3, which is commonly expressed in human airways, as a key human defence against avian flu.
The statement adds that through a series of extensive tests, the study team were able to show that the BTN3A3 gene is vital to protecting humans against avian flu, as most strains of the virus cannot get past its defences.
Avian flu, also commonly referred to as bird flu, primarily spreads among wild birds such as ducks and gulls and can also infect farmed and domestic birds such as chickens, turkeys and quails. Since 2022 there has been a rise in bird flu cases around the world in both domestic and wild birds.
While the disease mainly affects birds, it has been known to spill over into other species, including, in rare cases, humans. For example, the 1918 Spanish flu virus which caused more than 25 million deaths worldwide is believed to have originated from an avian strain.
‘‘However, experts agree there are still several gaps in our scientific knowledge that make it difficult to be able to predict which variant of avian influenza virus might spill over into the human population and when,’’ says the statement by the university.
Keen to know why some avian flu transmission does occur in humans, the team behind this important study compared the behaviour of hundreds of genes normally expressed by human cells during a viral infection with either human seasonal viruses or avian flu viruses. The study showed that the BTN3A3 gene was able to block the replication of avian flu in human cells. In contrast, the seasonal human flu viruses, which infect the human population regularly, are resistant to BTN3A3 meaning it cannot successfully block them.
The team also looked at avian flu viruses that occasionally do infect humans, for example H7N9, which since 2013 has infected more than 1,500 individuals with 40 pc case fatality rate. Researchers were able to show that avian flu viruses like H7N9 have a genetic mutation that allows them to ‘escape’ the blocking effects of the BTN3A3 gene.
Finally, when studying the evolution of avian flu strains, the scientists were also able to show that there had been increase in the number of BTN3A3-resistant strains circulating in poultry around the same time as spill over events in humans.
“We know that most emerging viruses with human pandemic potential come from animals. It is therefore critical to understand which genetic barriers might block an animal virus from replicating in human cells, thereby preventing infection. Of course, viruses are constantly changing and can potentially overcome some of these barriers by mutating over time. This is why virus genetic surveillance will be crucial to help us better understand and control the spread of viruses with zoonotic and pandemic potential,” says Professor Massimo Palmarini, Director of CVR, leader of the study.
“Identifying BTN3A3 resistant variants when they first emerge in birds might help prevent human infections. Control measures against emerging avian flu viruses can be tailored specifically against those that are BTN3A3-resistant, in addition to other genetic traits known to be important for zoonotic transmission,” says Dr Rute Maria Pinto, the first author of this study.
“This interesting study illustrates an important piece of the very complex puzzle underpinning viral transmission between species, and shows the continued need for One Health research for infectious disease. This type of mechanistic scientific insight, coupled with genetic surveillance, can offer a window into future disease risks to inform public health planning,” says Dr Stephen Oakeshott, Head of Infections and Immunity at the MRC, part of UKRI.
Tracking the history of influenza pandemics in humans, the researchers were also able to link BTN3A3 resistance with key influenza virus types. All the human influenza pandemics, including the devastating 1918-19 global flu pandemic and the swine flu pandemic in 2009 were caused by influenza viruses that were resistant to BTN3A3. As result, this study suggests that having resistance to this gene may be a key factor in whether any flu strain has human pandemic potential.